Researchers are always working on better ways to treat mesothelioma and other cancers. But before they can introduce new treatments to the general public, they must advance their research through multiple phases, including human trials.
One of these clinical trials started recruiting volunteers at the end of March 2023. The trial aims to test the “safety, feasibility, and potential activity” of the new drug, SynKIR-110. Here’s what mesothelioma victims may want to know.
What is SynKIR-110?
SynKIR-110 is a dual-chain CAR T-cell therapy drug designed by Verismo Therapeutics. The company is a spinout from the University of Pennsylvania, and it has recently received FDA Fast Tracking to test the use of SynKIR-110 for mesothelioma patients.
What is CAR T-cell therapy?
What is FDA Fast Tracking?
The FDA grants its Fast Track designation to select researchers who are working to “treat serious conditions and fill an unmet medical need.” Researchers who receive Fast Track status for their treatments benefit from increased contact with the FDA, limiting the amount of time required to advance between phases. The FDA does not look for promising research. Instead, researchers such as Verismo must request Fast Track designation.
When do the human trials begin?
The first-in-human trials of SynKIR-110 are already underway. At the time of writing, however, they are still in the recruitment phase.
Who is eligible to participate?
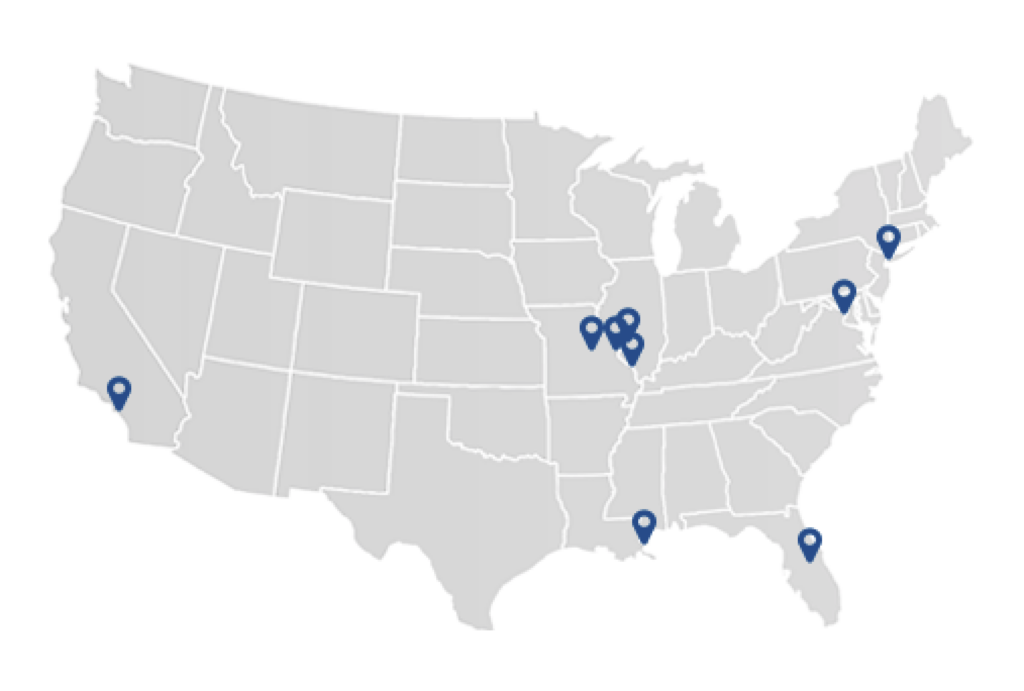
The trials are open to 42 volunteers. Mesothelioma victims looking to join must meet certain inclusion criteria, including:
- Must have already had one prior line of systemic therapy
- Age 18 or older
- Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1
- Tumor expression of mesothelin greater than or equal to 50% of tumor cells with greater than or equal to 2+ staining intensity
- Lesions measurable for mRECIST
- Satisfactory blood coagulation, plus satisfactory organ and bone marrow function
There are also several criteria that may exclude certain participants.
How can I sign up?
If you are interested in volunteering, you should first speak to your doctor. There are always risks associated with any medical treatment, especially new ones.
The application is available through ClinicalTrials.gov, where you may also find other information about clinical trials.